Article
Dermatological, Cosmeceutical and Corneotherapy: The difference explained
Dermatological, Cosmeceutical and Corneotherapy:
The difference explained
There is perhaps equal measures of misunderstanding and misconception regarding the grades of skincare products, particularly when the formulations approach dermatological effects.
This is not helped when new marketing terms are invented to imply performance. In this article we will look at the definition of the two main classes of products that straddle the border between cosmetic and medicinal and their use in Corneotherapy.
This article is a distillation and compilation of information sourced from colleagues who share my passion for sharing knowledge. FBH
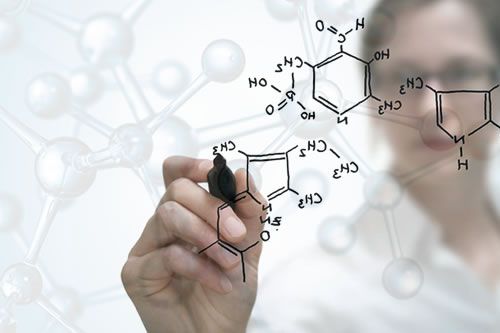

Cosmeceuticals: Cosmetic ingredients with pharmaceutical effects
The cosmetic industry uses the word Cosmeceuticals to refer to cosmetic products that have medicinal or drug-like benefits.
The term has been around for over 15 years, and is one of the new terms that is part of the new terms that evoke curiosity and encourage trial purchase of products. The terms “medical cosmetics”, “cosmedics” and “medicosmetics” are similarly circulated.
When these terms are borrowed or re-engineered from medicine or pharmacy language, you can be sure that the public will become interested in any new product that uses these expressions.
The terms dermatocosmetics, dermaceuticals, skinceuticals and cosmeceuticals were created specifically for this reason. If a manufacturer or marketer goes further and gets endorsement from individuals with the title Dr in their name, then this garnishes the brand name to reference a medical or scientific background. A scientific sounding name and a medical connection can almost guarantee excellent sales in many markets.


Let us be clear however: the term cosmeceuticals does not appear in the European Cosmetic Directive, nor is recognised by the American FDA.
Regardless of it’s legal status or recognition, it makes sense to define the term, particularly if a certain level of quality and performance is associated with it. So when can we, or should we speak of a cosmetic ingredient that has a pharmaceutical effect, AKA Cosmeceutical?
These are the recognized criteria:
1. The ingredient should not be listed among the banned substances in the European Cosmetic Directive.
2. Will not have any systemic effects. Example: Hormones are banned in cosmetics.
3. The active agent needs to be capable of penetrating into the skin barrier and further permeating into the epidermis as the target destination.
4. The destination (cell, tissue, blood vessel, enzyme, receptor, etc) and the triggered, intervened or inhibited biochemical process there should be proven both in vitro and in vivo.
5. The externally visible and advertised effect has to be clinically evident, statistically proven, reproducible and significant.
6. The product safety of the substance (about its toxic profile according to the requirements of the EU Cosmetic Directive) has to be documented in the safety report.
7. The ingredient will be a pharmaceutically active agent that improves and stabilises the condition of the skin and eliminates skin disorders.
8. Pharmaceutical claims such as skin healing, acne treatment, inhibition of fibrinolysis and antimycotic effects, etc, are not permitted for marketing and promotional purposes; even if they are evident with the use in cosmetic formulations.
If the criteria mentioned above strictly interpreted, many highly praised and modern cosmeceuticals would just fall through the cracks– either as a single substance or in a preparation combined with other substances.
As this type of formulation is not designed to act on the skin’s surface but destined to be effective in the deeper layers of the skin, cosmeceuticals should in principle be free of any counterproductive additives. Consequently, the inclusion of fragrance and allergenic preservatives do not fit the true concept of a cosmeceutical.
Equally; so as to avoid a washout of active agents and skin lipid components, non-degradable emulsifiers would be taboo in the case of products to treat skin barrier disorders.
With cornification disorders such as acne, the use of paraffin oils, and comedogenic hydrocarbons in a formulation does not correspond with cosmeceutical principles. While lipids should be avoided in formulations designed for perioral dermatitis, minor doses are allowed with rosacea.
In summary, the matrix of a true cosmeceutical formulation should guarantee an excellent ease of use, correspond with the physiology of the skin, be free of unnecessary additives and avoid adverse side-effects.
It can be said that these are the same, albeit often disregarded claims that are used for topical pharmaceuticals; a fact that has contributed to the creation and justification of cosmeceuticals.
It can be concluded that while cosmeceutical products have fewer restrictions regarding the selection of their matrix components, they are in no way inferior to pharmaceuticals.

While European skin care manufacturers have to comply with the EU Cosmetic Directive, in the US and different countries of South East Asia particular products, such as sun protection products, fall within the definition of “medical skincare”. These products are submitted to a separate, complex and costly registration process. The authorities in charge also routinely control the content of UV filters.
The close monitoring results from the fact that damage to the skin can still occur when application dosages are disregarded and consumers rely on the information on the labels.
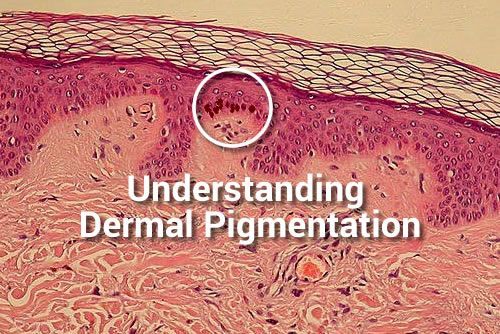
Dermatological: Cosmetics with dermatology affect
The term “dermatological cosmetics” already suggests the combination of dermatology and skin care, but what does dermatological cosmetics actually mean? What is the difference between dermatological and conventional cosmetics?
Based on a current examples, the concepts of dermatological skin and beauty care and their compatibility with existing laws and regulations can be described by the following:
The term “dermatological” is not a cosmetic treatment or activity that is comparable to the medical treatment provided by the dermatologist and consequently prohibited by law for use by cosmeticians, aestheticians, beauty therapists and skin treatment therapists.
The term “Dermatological” in connection with “dermatological cosmetics” however, represents a quality feature and describes a cosmetic treatment adapted to the physiological needs of an individual skin. (Cosmetics with dermatology affect).
For that reason, skin care concepts based on the types of creams used in dermatology (by a dermatologist) are finding their way in to cosmetic skin treatments (By appropriately trained skin treatment specialists) that are designed for intense skin care programs as well as supportive prevention strategies. This type of approach is gaining more and more importance in today’s world where skin barrier disorders are becoming more common.
These dermatological types of creams are known as Derma Membrane Structure (DMS), and can fulfil both the dermatological and supportive prevention requirements for skin, and as the name suggests, DMS creams are designed to be compatible to the physiological properties of the skin.
Skin treatment therapists use them as a base cream to apply separately (as a moisturiser) or in combination with cosmetically active agents, while dermatologists and pharmacists utilize them as a pure base cream for prescriptions, either individually or in combination with pharmaceutically active agents. From a chemistry point of view, the criteria for dermatological cosmetics are that they are free of non-physiological emulsifiers, preservatives, mineral oils, perfumes, dyes and additives. When we consider at the ingredients used in conventional cosmetics (INCI), these important criteria that make dermatological cosmetics true to their name cannot be ignored or taken for granted.
We have learnt in previous publications and education that the many emulsifiers that are not physiologically compatible to the skin can cause neurodermatitis and similar barrier disorders. As a matter of principle, any cosmetics designed from a dermatological approach should therefore be free of these emulsifiers. High concentrations of mineral oils and silicones in formulations may also impede the natural regeneration of the skin; studies of renowned scientists have proven this. Fragrances are mentioned as the number one sensitising substance in cosmetics today; and as with preservatives, individuals with a very sensitive or even pre-damaged skin are mostly affected.

The manufacturing costs of products free of preservatives and fragrance usually exceed the costs of producing conventionally preserved and fragrance-containing products simply because they must use much higher quality raw materials for manufacture. This is particularly apparent in water based creams, that require different ingredients and in particular, unscented raw materials. Due to these aspects and the ever rising costs of today, many manufacturers with a history of conventional formulations have too many cost barriers to allow them to produce an uncompromisingly dermatological product range. Consequently they are not as common as they could- or should be.
Unfortunately, the properties of the substances which show counterproductive effects after long-term treatment (and therefore should be avoided), are predominantly cited in dermatological spheres but far less frequently in cosmetic literature. The reason for this is obvious; for cosmetics marketers this type of information is “inconvenient” for unconditional acceptance of their products.
Dermatology deals with the needs of the skin itself. In all dictionaries and encyclopaedias the term “derma “directly refers to “skin” with dermatology explicitly and exclusively signifying skin science.
Consequently, another feature of dermatological cosmetics is a potential supportive care and prevention of skin disorders or even diseases. In this case, special emphasis is given on skin protection as described in the European guidelines which consequently results in additional quality features such as:
- Skin-identical or skin-related components.
- Creams with a physical structure identical to the membrane-like composition of the skin barrier layers.
Cosmetic dermatology or dermatological cosmetics complements the fields of both conventional medicine and traditional cosmetics/ skin care while extending their field of activity.
Aestheticians, skin treatment therapists, dermatologists and pharmacists with foresight already have realised customers needs, and are now expanding their range of services appropriately.
As a result, more and more therapists are seeking information on dermatology; while dermatologists who deal with cosmetics and pharmacists are improving their sales competence in Dermatics and cosmetics.
Dermopharmacy, cosmetic medicine, cosmeceuticals are the key words of today which refer to the fact that there is no longer a clear dividing line between skin care, prevention,and treatment. Furthermore, many of the substances are used as cosmetically and dermatologically active agents.
Two example of this are Urea and D-panthenol. Urea is an ingredient used in cosmetic formulations for dry, stressed skin and in dermatology in creams for the treatment of neurodermatitis. D-panthenol supports the regeneration of the skin in cosmetic applications and is used to accelerate healing in dermatology.
Corneotherapy : The link between cosmetics and dermatology
Corneotherapy with Dermatological cosmetics takes the science of cosmeceuticals one step further, and is rapidly becoming recognised as the modality that provides the link between dermatology and cosmetics. (Dermatological)
In this context, we can reasonably and correctly title the formulations used in true Corneotherapy as Corneotherapeutic.
Corneotherapy can be described as a modality or methodology that focuses on ameliorating (restructuring) the stratum corneum without using any pharmaceutical agents. Corneotherapy uses dermatological cosmetics based on the dermatological types of creams known as Derma Membrane Structure to obtain it’s efficacy.
The key feature of Corneotherapy is that the primary task of any formulation or treatment is to correct or create homeostasis in the skin so that it will then effect it’s own repair.
This is particularly significant with the increasing number of skin conditions related to, or exacerbated by barrier disorders of various types.
Without discussing the complicated biophysical processes in detail, in summary, it may be said that corneotherapy aims at a recovery of the stratum corneum and above all, that it improves the skin barrier function and consequently the homeostasis of the skin.
Corneotherapy is known for it’s signature outside-in therapy approach, whereas “outside” is the stratum corneum and “in” are the therapeutic effects starting in the stratum corneum and working their way into the deeper skin layers.
In contrast, there is conventional inside-out therapy where a pharmaceutical agent will inhibit inflammatory processes in the skin or influence the immune system while only having secondary effects on the stratum corneum (“out”). This comparison clearly shows the potential significance of an adjuvant care of the stratum corneum, even in combination with a conventional therapies.
In order for the outside-in therapy approach to work with minimum side effects, the formulations used must follow the same strict criteria as dermatological products. This would exclude many of the cosmeceuticals currently available because of their unnecessary additives and non physiological ingredients.
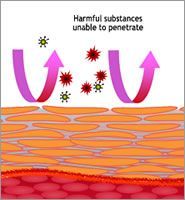
It is well established that a healthy and properly functioning skin barrier inhibits pathogenic germs, such as Staphylococcus aureus that is widely found in cases of atopic dermatitis, from penetrating into the epidermis. A disordered stratum corneum, however, tends to support recurrences. This example shows the importance of avoiding counterproductive effects of skin care that has not been created with skin barrier health in mind.
It has been known for quite some time, that skin cleanser components such as sodium lauryl sulfate (SDS) may cause major skin irritations by firstly damaging the barrier function and subsequently causing cellular reactions.
Furthermore, a SDS damaged skin barrier enables sensitizing substances that are used to preserve and fragrance skin care products, to easily penetrate the skin. Interestingly, SDS is widely used as a standard irritant in dermatological research.
One of the major advantages of corneotherapy using dermatological cosmetics is that it is largely free of side effects in comparison with a treatment with topical pharmaceuticals.
Preventively applied corneotherapy may well extend the intervals between occurrences and reduce; or even avoid the application of conventional dermatic products.
A precondition for successful corneotherapy, however, is a precise diagnosis of the skin and relevant conditions, and the application of expertise regarding the structure of skin care products and their components.
Besides the above mentioned moisturizing substances, lipids, and filming agents, ceramides, as well as amides play a significant role in the selection of components.
Despite the effectiveness of Corneotherapy in the treating of many skin barrier related conditions and beyond, there is only hope that it will (despite its relatively unspectacular procedures compared with conventional treatments) may soon gain acceptance.
Compilation, translation and editing by Florence Barrett-Hill.
References
1. Dr Hans Lautenschläger; Dermatological cosmetics – linking cosmetics and medicine published in Kosmetische Praxis 2005 (5), 12-14 http://www.dermaviduals.com/english/publications/products/dermatological-cosmetics-linking-cosmetics-and-medicine.html
2. Dr Hans Lautenschläger; Highly effective – Cosmeceuticals- published in medical Beauty Forum 2014 (4), 16-18
3. Lautenschläger H, The history and current aspects of corneotherapy, IV. International Symposium on Aesthetic Medicine, Moscow, April 19-20th, 2005
4. Friberg SE et al., Water permeation of reaggregated stratum corneum with model lipids, J Invest Dermatol 1990;94:377-380
5. Lübbe J, Secondary infections in patients with atopic dermatitis, American Journal of Clinical Dermatology 2003;4(9):641-654
Addendum;
The United States (US) and European Union (EU) both work to ensure the safety of cosmetics for consumers through rigorous regulation.
The 27 European Union Member States have transposed the European Union Cosmetics Directive, enacted in 1976, into national law. Each Member State has health authorities that then regulate cosmetics within their respective national boundaries according to the law.
In the United States, the cosmetics industry is regulated by the U.S. Food and Drug Administration (FDA) which has been granted broad regulatory authority under the Federal Food, Drug, and Cosmetic Act, enacted in 1938.
See more at:
http://www.cosmeticsinfo.org/
The post Dermatological, Cosmeceutical and Corneotherapy: The difference explained appeared first on Pastiche .
share this
Related Articles
Related Articles